4. Xenovirology
4.1. Discovery of the Molecular Mechanism of how Low-Nanomolar TCDD Triggers HIV-1 trans-activation in Human Target Cells.
In a deviation from the mainstream studies of dioxin effects on vertebrate species, Tsyrlov with Andrey Pokrovsky (in the photo)

of the State Research Center of Virology and Biotechnology VECTOR made a pioneering finding on transactivation by TCDD of the HIV-1 in target human T-lymphocytes.[49],[50],[51] Specifically, using HIV-1-infected CD4+ cells, instead of highly anticipated toxic effects of 1.0-10.0 nM TCDD, a several-fold increase of viral reverse transcriptase (RT) activity, HIV-1 antigen, and a substantial activation of Hiv long terminal repeat CAT construct were shown. The dose- and time-dependent induction of CYP1A1 in the host cells and the inhibitory effect of monoclonal antibodies raised against CYP1A1 were demonstrated. So, the conclusion was drawn that TCDD effects registered in CD4+ T-lymphoid cells are mediated via the AhR/(bHLH)/Per-Arnt-Sim(PAS domain) transcriptional pathway.

The revelation that the AhR has a role in mediating transactivation of human HIV-1 has been thereafter confirmed in several separate labs (summarized in[52]). However, until its revival in the early 2000s, Tsyrlov's finding did not have any development, just remaining a sui generis observation due to lack of mechanistic knowledge of dioxin action on human DNA viruses, and because 2,3,7,8-TCDD concentrations used with HIV-1 were three orders of magnitude higher than dioxin body burden in the general human population.
REFERENCES
[49] Pokrovsky, A.G., Chernykh, A.I., Yastrebova, O.N., Tsyrlov, I.B. (August 1991). “2,3,7,8-tetrachlorodibenzo-p-dioxin as a possible activator of HIV infection”. Biochemical and Biophysical Research Communications 179:46-51. ISSN: 0006-291X
[50] Tsyrlov, I.B., Pokrovsky, A.G. (August 1993). “Pleiotropic trans-activating effects of TCDD, B[a]A and B[a]P on structural genes of CYP1A1 and HIV-1 in human CD4+ lymphoid cells”. Organohalogen Compounds 13:179-182. ISSN:1026-4892
[51] Tsyrlov, I.B., Pokrovsky, A.G. (September 1993), “Stimulatory effect of the CYP1A1 inducer 2,3,7,8-tetrachlorodibenzo-p-dioxin on the reproduction of HIV-1 in human lymphoid cell culture”. Xenobiotica 23:457-467. DOI: 10.3109/00498259309057034
[52] Tsyrlov, I.B. (July 2006). “ The role of body burden 2,3,7,8-TCDD in triggering malignancy-associated human viruses: from early data to mechanistic concept”. Organohalogen Compounds 68:552-555. ISSN:1026-48924.2. Expression of Human Hepatitis B Virus and Gene of Influenza A virus non-Structural 1 Binding Protein Might be trans-Activated with TCDD in the Sub-Nanomolar Range.
4.2.1.
Two sets of information appeared in 2002 spotlighting a path to the underlying mechanism of Tsyrlov's earlier data on the TCDD effect on human DNA viruses. First, Tim Zacharewski's lab at the University of Michigan presented a technical report entitled “Species DRE Summary” (http://www.bch.msu.edu/~zacharet/resources/species_dre_summary.html). Therein, an in silico tool was used to determine the substitution intolerant DRE core sequence motif 5’-GCGTG-3’ in various eukaryotic promoters, including gene promoters of some human viruses. The most intriguing was that multiple 5’-GCGTG-3’ core sequences were mapped in the 5' flanking region of Epstein-Barr virus (EBV), Hepatitis B virus (HBV), Cytomegalovirus (CMV), and Herpes simplex virus, type 1 (HSV-1). Second, a strong up-regulation of CMV gene in host human cells was shown in the presence of TCDD at a concentration lower than its background level determined in the general population.[53] The involvement of the AhR and AhR nuclear translocator (ARNT) in transactivation of the virus replication was also demonstrated. To develop properly the mechanistic concept of chemico-biological interactions between bodily TCDD and human dormant viruses,[54] Tsyrlov's team initially verified the data on viral DRE from the Zacharewski lab. As a weight matrix method utilized by the Michigan team relies upon the largely unproven assumption that the nucleotides of binding sites exert independent effects on binding affinity, and is characterized by recognition threshold MS values lower than an acceptable threshold, the SITECON tool was used for counting the number of potentially active promoter DRE in the viral genes. Alongside with Michigan team, the only promoter DRE was confirmed in HIV-1 gene and in HPV-16 gene. However, with the recognition threshold of 0.95, SITECON identified 5 to 10 active DREs in regulatory regions of five CMV genes, 2 to 3 DREs in HBV gene, and 7 to 8 DREs in genes encoding major proteins of HSV-1. The above directly applied to Tsyrlov' idea that the level of susceptibility of a viral gene to DLC depends on the number of potent DRE motifs within the gene' regulatory region, similar to what Yoshiaki Fujii-Kuriyama revealed with the mammalian gene models containing DREs in upstream activating sequence. Also, a mammalian cell-based DRESSA bioassay system developed for detection of at least 0.5 pM TCDD, contains tandem copies of the DREs fused to a minimal viral promoter, and subcloned into an expression plasmid upstream of the reporter gene.[55] The concept explains why possessing the only promoter DRE the HIV-1 gene was transcriptionally activated by non-physiological (high) TCDD concentrations of 1.0-10.0 nM.
EPA' Lecture July 25 2006 (5).pdf
4.2.2.
TCDD is the reference chemical to which the toxicity of other dioxins and dioxin-like compounds (DLCs) are compared. Decreasing trends in dioxin dioxin body burden (DBB) over four decades in blood and adipose tissue of the general population of the United States culminated in its current serum level of about 2-4 ppt (pg/g).[56] Therefore, juxtaposing 5-10 potent core DRE motifs in the promoter region of CMV vs the only promoter DRE in HIV-1 to corresponding potent transactivation concentrations of TCDD, it was suggested that the hepatitis B virus (HBV) gene possessing 2 to 3 promoter DREs might be upregulated in human liver cells by TCDD at level of the same order of magnitude than the DBB. So, multidisciplinary approach to the trans-activation of human Hepatitis B Virus by body burden dioxin or dioxin-like polychlorinated biphenyls was undertaken. Background Human viruses evolved into novel target genes for host cell dioxin receptor (AhR/Arnt) transcriptional complex since the discovery of trans-activation of HIV-1 by 1.0 nm 2,3,7,8-TCDD (dioxin), the most potent xenobiotic with extremely long half-life in humans. Later, up-regulation of cytomegalovirus (CMV) in human cells was shown with 0.3 pm dioxin, lower than current background level in the general population (4–6 pg/kg). However, the mechanism of viral trans-activation by dioxin was revealed after “dioxin response elements” (DRE) were computationally identified in viral promoters: a single DRE in HIV-1 and 8–10 DRE in CMV. Here, the compilation of experimental, in silico, epidemiological, and medical prospective data, all concerning effects of dioxin on the HBV are presented.[56, a] Materials and Methods Production of the HBV in HepG2 cells was determined using plaque assay, viral DNA – by hybridization and PCR. A computational search for DRE in HBV genes performed by the SITECON. The latter identified 2 to 4 promoter DRE in the HBV. Juxtaposing the DRE numbers with experimental results suggested that the HBV might be upregulated by dioxin at concentrations lower than 1.0 nm and higher than 0.3 pm shown for the HIV-1 and CMV, respectively. If fact, the lowest effective concentration was 70 pm. That is in the range of bodily dioxin level of some overexposed population groups in the Arctic and Vietnam consuming dioxin-contaminated seafood, resulted in high prevalence rates of chronic hepatitis B and HCC. Such elevated body content of TCDD has been reported for some Arctic and South-Eastern Asia populations. In a compiled study, the effects of TCDD on the HBV were evaluated. Experimentally, the production of the HBV in HepG2 cells and viral DNA were both nine to tenfold elevated by 50.0 pM of TCDD. Epidemiologically, a high rate of viral hepatitis B is found in the Arctic and Vietnam populations that regularly consume dioxin-contaminated fat in seafood thus demonstrating how environmental dioxins biomagnify up the food chain. Viral hepatitis B outbreaks, in turn, resulted in high prevalence rates of hepatocellular carcinoma (HCC) in the Arctic and some provinces in Vietnam. The same defines increased mortality from cirrhosis and HCC among PCB-exposed ‘Yucheng’ patients in Taiwan, which is the HBV-endemic area. Conclusions All the above provide evidence for activation of DRE-containing HBV with sub-nanomolar dioxin, and allow exploring inhibitors of HBV-driven malignancy among the AhR antagonists or modifiers of AhR/Arnt complex binding to viral DRE.[57]

With Dr. Linda Birnbaum, a worldwide leading expert on dioxin toxic effects. In 2009, Dr. Birnbaum became the director of NIEHS/NIH.
4.2.3.
Following the causes and consequences of the global spread of Influenza A Virus strain H5N1 that occurred in 2005–2007, Tsyrlov with Vladimir Roumak conducted a complex study on Influenza A Virus non-structural 1 binding protein (NS1) in human and avian host cells,[58], [59] as the NS1 is known to prevent transcriptional induction of antiviral interferons. In silico, the NS1 gene was shown to possess 2 potentially active promoter DREs. Experimentally, infected HeLa cells were treated with 40.0 pM TCDD and revealed several-fold intensive NS1-specific polypeptide. On the epidemiological level, the data showed that wild birds and domestic poultry (G. gallus gallus) were dying from H5N1 in those provinces of China and Vietnam where water and soil were highly contaminated with DLCs. Because alveolar epithelial cells is a centerpiece of seasonal Influenza A virus-caused lung injury, and an increased NS1 polypeptide is detected in TCDD-treated human epithelial cell line,[60] a conclusion was drawn that within human cohorts from regions slightly overexposed to DLCs, the NS1 might serve a promotional factor for seasonal influenza epidemics.
4.3. Human Herpesviruses Possessing Multiple Promoter DREs Might be trans-Activated with TCDD in the Low-Picomolar Range.
Due to the omnipresence of dioxins, all people have background exposure but current normal level of dioxins in the body, so-called body burden, formally is not expected to affect human health on average.[61] However, Tsyrlov and others bioinformatics, experimental and epidemiological data with DRE-containing human DNA viruses shed novel light on the subject and transformed lately into a mechanistic concept of Xenobiotical Virology.[62] The concept has been epitomized in human herpes viruses containing multiple DREs, such as the CMV, HSV-1, and EBV. The complex study on EBV is still in progress, so the burden of proof lies with the former two common human viruses. Earlier the SITECON detected 5 to 10 active DREs in regulatory regions of five CMV genes, and a sub-picomolar TCDD was shown at Tsugiya Murayama lab to augment replication of the CMV in human fibroblast cell line. As the CMV is merely an epiphenomenon of inflammation in macrophages, Tsyrlov with Jerome Wu revealed up-regulation effects of TCDD in the picomolar range on CMV in human macrophage cell line.[63] Also, DREs in regulatory regions of genes encoding macrophage inflammatory proteins and anti-inflammatory cytokines and cell factors were assessed.[64] Special attention was paid to p65, as within transcription factor NFκB the Rel family members of DNA-binding proteins, the (p65) mediates the most potent gene activation. That is because the ''p65'' possesses nine promoter DREs, three of those, at positions -110, -137, and -199, are each characterized by a MS score greater than 0.95, which makes the p65 gene the strongest candidate for transactivation by TCDD in the picomolar range. Another common virus, the HSV-1 is a human pathogen with worldwide seroprevalence rates ranging from 50% to 90%, which causes diseases ranging from simple cold sores to lethal encephalitis. However, factors initiating in rare situations the expression of HSV-1 lytic cycle from the latent viral genome leading to generalized infection are still not known. As the first line of defense against HSV-1 infections involves the production of interferon α and Interferon β1a and recruitment of cytokines and macrophagal transcriptional factors, Tsyrlov with Darmara Furman computationally assessed promoter DREs in HSV-1 genes, as well as genes of macrophagal antiviral proteins.[65]
Within five HSV-1 genes, including critical immediate early gene IE-IV/V, the SITECON detected 7 to 8 active DREs in their regulatory regions. It was shown that among responding to HSV-1 cytokines and macrophagal transcription factors genes encoding RANTES/CCL5, NF-κB3/p65/RelA and STAT3 possess seven to nine active 5’upstream DREs, which makes possible that sub-nanomolar TCDD causes the production of the HSV-1 and pro-inflammatory cytokines production via host cell AhR/(bHLH)/Per-Arnt-Sim(PAS) transcriptional pathway. Because genes of chemokines RANTES and STAT3 each contain 7 to 9 promoter harboring DREs, a new level of control of cellular functions by HSV-1 was suggested including persistent NF-κB3/p65/RelA activation in HSV-1 infected cells, rather than p65 being a host cell only response to the virus. Regarding the STAT3, its elevated signaling was proposed to promote oncolytic HSV-1 replication, likely by means of inhibiting the IFN-γ response.
REFERENCES
[53] Murayama, T., Inoue M., Nomura, T., Mori, S., Eizuru Y. (August 2002). “2,3,7,8-Tetrachlodibenzo-p-dioxin is a possible activator of human cytomegalovirus replication in human fibroblast cell line”. Biochemical and Biophysical Research Communications 296:651-656. DOI: 10.1016/s0006-291x(02)00921-x
[54] Tsyrlov, I.B., Shur, I.N. (August 2008). "Mechanistic Insight into Activation by Dioxin-Type Xenobiotics of Cancer-Linked Common Human Viruses and HIV-1 Virus". The Lancet Infectious Diseases 8:P496-9. DOI:10.1016/70172-5
[55] Kasai, A., Hiramatsu, N., Hayakawa, K., Yao, J., Kitamura, M. (March 2008). “Direct, Continuous Monitoring of Air Pollution by Transgenic Sensor Mice Responsive to Halogenated and Polycyclic Aromatic Hydrocarbons”. Environmental Health Perspectives 116:349-354. DOI:10.1289/ehp
[56] Lorber, M. A. (October 2002). "Pharmacokinetic Model For Estimating Exposure Of Americans To Dioxin-Like Compounds In The Past, Present, And Future". The Science of the Total Environment 288:81–95. https://digitalcommons.unl.edu/usepapapers/104 PII:S0048-9697Ž01.01119-6
[57] Tsyrlov, I.B., Pokrovsky, A.G., Wu, J., Shur, I.N. (September 2015). “Multidisciplinary approach to the trans-activation of human Hepatitis B Virus by body burden dioxin or dioxin-like polychlorinated biphenyls”. Journal of Viral Hepatitis 22:27-28. Online ISSN:1365-2893
[58] Wu, J., Pokrovsky, A.G., Rumak, V.S., Tsyrlov, I.B. (July 2007). “Regulation of human gene of Influenza A virus-associated NS1-binding protein by 2,3,7,8-TCDD: mechanistic data and epidemiological findings”. Organohalogen Compounds 69(2):1885-1889. ISSN:1026-4892
[59] Tsyrlov, I.B., Shur, I.N., Wu, J., Roumak, V.S. (September 2016). “A Decade Later, Another Look at What Role in 2005-2007 Global Spread of H5N1 Played an Up-regulation by Host Cell Dioxin of Gene Encoding Type A Influenza Virus NS1 Binding Protein”. OA Journal of Infectious Diseases and Therapy 4:133-8. DOI:10.4172/2332-0877.C1.013
[60] Tsyrlov, I.B., Shur, I.N., Roumak, V.S. (August 2017). “The NS1A binding protein in alveolar epithelial cells as centerpiece of Influenza virus A lung injury mechanism mediated via AhR/Arnt signaling pathway”. European Respiratory Journal 50:PA352. DOI:10.1183/1393003.congress-2017.PA352
[61] (October 2016). "Dioxins and their effects on human health". WHO Factsheets Newsletters. https://www.who.int/news-room/fact-sheets/detail/dioxins-and-their-effects-on-human-health
[62] Tsyrlov, I.B., Shur, I.N., Oshchepkov, D.Yu., Pokrovsky, A.G. (June 2012). "Xenobiotical virology: novel mechanistic concept of hazardous human viruses up-regulation by body burden level of dioxins via AhR-mediated transcriptional pathway". International Journal of Infectious Diseases: IJID: Official Publication of the International Society for Infectious Diseases vol. 16(Suppl. 1):e115-119. DOI:org/10.1016/j.ijid.2012.05.258.e112:
[63] Wu, J., Shur, I.N., Tsyrlov, I.B. Current human body burden of dioxins might upregulate DRE-containing cytomegalovirus (CMV) linked to inflammation and malignancy pathways. (July 2008) Organohalogen Compounds 70: 1471-1474 (2008). ISSN:1026-4892
[64] Oshchepkova, E.A., Oshchepkov, D.Y., Katokhin, A.V., Furman, D.P., Shamanina, M.Y., Mordvinov, V.A., Tsyrlov, I.B. (July 2008). “Regulatory regions of human genes encoding macrophagal transcription factors possess multiple potential dioxin response elements”. Organohalogen Compounds 70:1467-1470 (2008). ISSN:1026-4892
[65] Tsyrlov, I.B., Shur, I.N., Wu, J., Furman, D.N., Oshchepkov, D.Yu. (August 2014). A proposed mechanism of transactivation by body burden dioxin of Herpes Simplex Virus genes might be responsible for weakening the host antiviral defense”. Organohalogen Compounds 76:2b9-292. ISSN:1026-48924.4. Body Burden TCDD May Play a Role in Pathogenesis of Alzheimer Disease by trans-Activation of HSV-1 in Glial Cells.
It has been indicated in 100+ publications by many groups that the neurotropic HSV-1 might be a causative element of Alzheimer’s disease and mental retardation in elderlies.[66] No distinct factor responsible for reactivation of HSV-1 is revealed yet. While this dormant herpes virus lytic cycle activation in the brain typically linked to stress, immunosuppression, or inflammation, the extremely potent chemical factor in human body has been overlooked, namely TCDD, traces of which exist in human brain tissue. To address the point, Tsyrlov created the Project dedicated to Alzheimer’s disease.[67],[68],[69] It is based on reactivation of dormant human viruses by traces of TCDD, the most potent xenobiotic so far know. TCDD is shown to bioaccumulate progressively during maturation and aging) in human tissues, and its estimated half-life in humans is about 10 yr. This is the worst-case scenario of chemico-biological interactions, which supposes that "dormant human xenobiotic” TCDD is able to trans-activate the HSV-1 gene via host cell bHLH/PAS transcriptional complex. Presumably, binding of the activated complex at the viral promoter harboring DRE might occur while the HSV-1 progeny is formed in the nucleus of the infected cell, and viral DNA is packed into preformed capsids. TCDD at 1.0-5.0 pM was shown to cause a multifold elevation of HSV-1 titer and viral DNA content in HSV-1 infected murine Apoe(-/-) astrocytes and human astrocytoma U-87MG cells.[70],[71]

So, reactivation of HSV-1 was demonstrated with TCDD concentrations close to the current dioxin body burden level in the general population. The astrocytoma cell lines were chosen because earlier amyloid plaques and their development during Alzheimer’s disease intimately linked to activated astrocytes. Epidemiological outlook has noticed the relationship of a higher rate of Alzheimer’s disease among native Arctic populations, and elevated dioxin body burden due to regular consumption of sea mammal fat contaminated with TCDD and DLC. Also, Tsyrlov and Krivoschokov showed up-regulation of the brain inflammatory cytokines.[72] Genes of those cytokines possess multiple promoter DREs, which implicates inflammation to Alzheimer’s disease pathogenesis, especially in the elderly people pre-exposed to dioxins thru the marine food chain. So, multiple promoter DREs in HSV-1 and a remarkable sensitivity of HSV-1 in target brain cells to picomolar concentrations of TCDD, all make this dormant neurotropic virus to become the target for TCDD in brain tissues. Supposedly, in each and every case the real chance of latent HSV-1 reactivation by TCDD in brain cells is determined by the AhR affinity to TCDD, which varied ~15-fold in human populations.[73]
REFERENCES
[66] Itzhaki, R.F. (August 2017). “Herpes simplex virus type 1 and Alzheimer’s disease: possible mechanisms and signposts”. The FASEB Journal. Alzheimer’s Disease Review Series 31:3216-3226. DOI: 10.1096/fj.201700360
[67] Tsyrlov, I.B. (January 2010). Project: “Xenobiotical Virology: Mechanistic Insights, Epidemiological and Clinical Implications”. https://www.researchgate.net/profile/Ilya_Tsyrlov
[68] Tsyrlov, I.B., Wu, J., Shur, I.N., Oshchepkov, D.Yu. (July 2016), “Alzheimer’s disease causative viruses HSV-1 and CMV are highly susceptible targets for bHLH/PAS dioxin receptor-Arnt transcription pathway in glial cells”. Alzheimer's and Dementia. The Journal of the Alzheimer Association 12(7):P632-633. DOI:10.1016/j.jalz.2017.06.2252
[69] Tsyrlov, I.B., Shur, I.N., Appelbaum, J., Wu, J. (June 2017). “AhR-ARNT signaling pathway mediates transcriptional activation of HSV-1 in glial cells: a pathogenic factor that might affect AD development”. Alzheimer's and Dementia. The Journal of the Alzheimer Association 13(7):P1438-P1439. DOI: 10.1016/j.jalz.2017.06.2252
[70] Tsyrlov, I.B., Wu, J., Shur, I.N., Oshchepkov, D.Yu. (July 2016), “Alzheimer’s disease causative viruses HSV-1 and CMV are highly susceptible targets for bHLH/PAS dioxin receptor-Arnt transcription pathway in glial cells”. Alzheimer's and Dementia. The Journal of the Alzheimer Association 12(7):P632-633. DOI:10.1016/j.jalz.2017.06.2252
[71] Tsyrlov, I.B., Shur, I.N., Appelbaum, J., Wu, J. (June 2017). “AhR-ARNT signaling pathway mediates transcriptional activation of HSV-1 in glial cells: a pathogenic factor that might affect AD development”. Alzheimer's and Dementia. The Journal of the Alzheimer Association 13(7): P1438-P1439. DOI: 10.1016/j.jalz.2017.06.2252
[72] Tsyrlov, I.B., Krivoschekov, S.G. (July 2016). “Higher rate of Alzheimer’s disease among Arctic populations is due to activation of brain inflammatory pathways by elevated dioxin body burden: mechanistic approach to the problem”. Alzheimer's and Dementia. The Journal of the Alzheimer Association 12(7): P3-112-114. DOI:10.1016/j.jalz.2016.06.1770
[73] Harper, P.A., Wong, J.Y., Lam, M.S.M., Okey, A.B. (September 2002). "Polymorphisms in the human AH receptor". Chemico-Biological Interactions 141:161-87. DOI:10.1016/s0009-2797(02)00071-64.5. Epidemiological Approach to Higher Rate of Alzheimer Disease in Populations Possessing Elevated Body Burden of TCDD-like Compounds.
While Alzheimer’s disease (AD) affects millions globally, its pace prevails in Arctic populations. Thus Finland has the highest incidence of AD, Iceland and Sweden aren’t far behind, and in northern Canada AD dementia incidence rate in adults age 45+ increased by 2.8 to 5.1 times every 10 years(1). Meanwhile, a significant airborne transfer of dioxin-like compounds to the high latitudes has been found (2), and GC/MS measurements reveal in Arctic natives a 7 to 25 times higher body burden of dioxins (BBD) than current BBD (3 ppt) in general population in middle latitudes areas(3). A prototypic dioxin, extremely hydrophobic TCDD increasingly accumulates through the marine food chain thus making Arctic people exposed to larger concentrations of dioxins because their traditional diet includes sea mammal fat.
As astrocyte-derived pro-inflammatory cytokines play pathogenic role in AD(4), and their gene promoters possess “dioxin responsive elements” (DRE)(5), effect of elevated BBD on cytokines expression were studied in silico and by immunoassays. The cytokine expression profile in untreated and TCDD treated (10 ppt, 48hr) human astrocytes (DTHA) was evaluated by using a protein microarray. Pre-stimulated with IL-1β and TNF-α, cultured astrocytes expressed pro-inflammatory cytokines IL-6, IL-1β, TNF-α, and RANTES (CCL5). Expression of pSTAT3 in astrocytes was measured by Western blotting. Computational tool SITECON was used to detect and quantify DRE, the substitution intolerant core sequence (5’-GCGTG-3’) and adjacent variable sequences. Earlier we used SITECON to in silico identify the sites with functionally active DRE in mammalian(6) and viral genes(7) at the recognition threshold of 0.95. In DTHA, different levels of cytokines expression were detected, namely IL-6, IL-1β, TNF-α – by 2.0-2.5 times, and IL-12, RANTES, STAT3 – by 4.5-5.0 times the control level. Computationally, genes encoding IL-1β, IL-6 and TNF-α each contains just one or two promoter DRE, whereas genes encoding IL-12, RANTES, and STAT3 possess four, nine and eight potentially active DREs, respectively. The abundance of promoter DRE makes those genes extremely susceptible to AhR/Arnt-mediated transcriptional activity(5-7) corroborated by higher expression level of IL-12, RANTES, STAT3. It is noteworthy that nine DRE was also determined in gene encoding the transcription factor NFkB, which expression multiplied 5 times in DTHA. This study shows up-regulation of brain inflammatory proteins thus further implicating inflammation to AD pathogenesis, especially in people overexposed to dioxin-like compounds.
REFERENCES
(1) Eiser, T. Why does Finland have the highest dementia mortality rate? Environmental factors may be generalized. Brain Res. 2017, 1671, 14-17.
(2) Booth, S., Hui, J., Alojado, Z. Global deposition of airborne dioxin. Marine Pollut. Bull. 2013, 75, 1-2.
(3) Johansen, B. The Inuit's Struggle with Dioxins and Other Organic Pollutants. American Indian Quart. 2002, 26, 479-490.
(4) Villalba, M., Hott, M., Martin, C., et al. Herpes simplex virus type 1 induces simultaneous activation of Toll-like receptors 2 and 4 and expression of the endogenous ligand serum amyloid A in astrocytes. Med. Microbiol. Immunol. 2012, 201, 371–379.
(5) Tsyrlov, I.B., Wu, J.S., Oshchepkov, D.Y. Transactivation by body burden dioxin of Herpes Simplex Virus genes and cytokines genes can weaken the host antiviral defense. MO Journal Cell Science & Report. 2017, 4, 89-95.
(6) Tsyrlov, I.B., Shamanina, M., Furman, D., Oshchepkov, D.Y. In silico approach for discovering functional DREs in regulatory region in human genes encoding members of Ah receptor signaling pathway. Drug Metabolism Reviews 2016, 48, 4-6.
(7) Tsyrlov, I.B., Shur, I.N., Oshchepkov, D.Y. Human viruses: A novel target for bHLH/PAS dioxin receptor-Arnt transcription factor. J. Antivirals and Antiretrovirals. 2016, 8 (5), 42-44.4.6. Dioxin in the picomolar Range in Lungs May Trigger Cytomegalovirus Reactivation Among Severe COVID-19 Patients with Acute Respiratory Distress Syndrome.
Tsyrlov, I.B., Shur I.N. Reactivation of CMV and HSV-1 Among Severe COVID-19 Patients May be Triggered by Increased Level of Pulmonary Dioxin. International Journal of Infectious Diseases ( 2022) 116:S59-61. DOI:10.1016/j.ijid.2021.12.139 PMCID: PMC8884754
Tsyrlov, I.B., Shur, I.N. Rising level of pulmonary dioxin might trigger CMV and HSV-1 reactivation via bHLH/PAS/ AhR:ARNT transcription pathway in lungs of immunocompetent COVID-19 patients with ARDS. Journal of Biological Chemistry (2023) 299(3): 50S-51S. https://doi.org/10.1016/j.jbc.2023.103163A multi-parameter clinical and epidemiological information collected and analyzed by Irina Shur, M.D. (in the photo), currently

a Medical Director at the Montefiore Einstein Advance Care (MEAC) center in Elmsford (New York), showed that reactivation of human cytomegalovirus (CMV) infection influenced worse clinical outcomes following SARS-CoV-2 infection.(1) CMV and herpes simplex virus 1 reactivation were found to be frequent events in patients with COVID-19 acute respiratory distress syndrome (ARDS), and Herpesviridae detection in the lower respiratory tract is associated with worse outcomes.(2) CMV reactivation may trigger a cytokine storm, thus playing a contributory role in individuals with severe COVID-19 disease.(3) Although secondary immunodeficiency and several stress factors are assumed to cause CMV reactivation, there is increasing evidence to suggest that CMV reactivation frequently occurs in critically ill immunocompetent patients and also in the absence of stress stimuli, so the molecular factor that triggers Herpesviridae reactivation is still not clear. At the same time, the biologically potent xenobiotic TCDD has been shown to be a mechanistically plausible cause of cell signaling pathways, resulting in an increase in CMV lytic genes expression and the production of viral particles.(4–7)
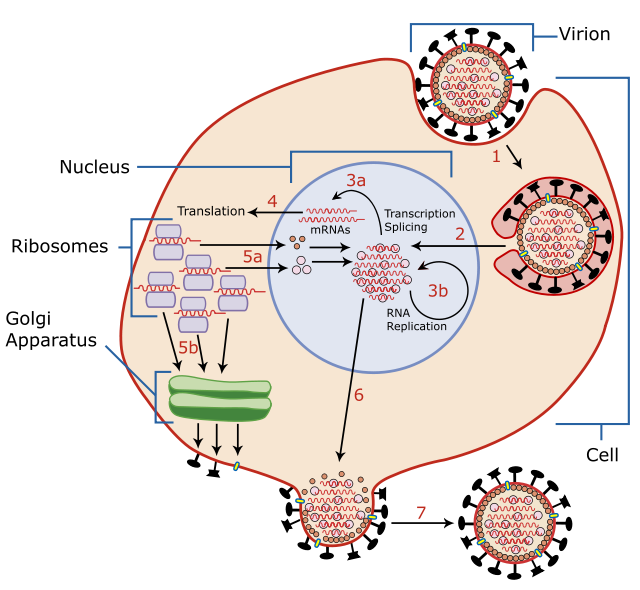
Substantiating the point, an integrated approach was used, combining experimental, bioinformatics, epidemiological studies, and clinical findings. TCDD accumulates with age in human fat tissue, biomagnifies through the food chain, has a half-life in humans of eight to ten years, and has a current body burden in the general population ranging from 5 to 20 pg/g (TEQ in fat) and 50 to 100 pg/g in older people. TCDD binds with high affinity to the cytosolic aryl hydrocarbon receptor (AhR) belonging to the basic helix-loop-helix-PER-ARNT-SIM family of proteins. Following ligand binding, the receptor rapidly translocates to the nucleus, where the heterodimeric TCDD:AhR:Arnt complex binds elements of DNA with the core sequence 5’-GCGTG-3’, known as dioxin-responsive elements (DRE), in the promoter of target genes. In the 2000s, a mechanism-based concept was developed due to experimental evidence of strong upregulation of CMV genes in human fibroblast(4) and macrophage cell lines(5,6) with TCDD at low picomolar (human body burden) concentrations. This resulted in an activated AhR:Arnt transcriptional complex binding major immediate-early promoter DREs within CMV genes.(7) To validate the concept with bioinformatics analysis, a proven computational tool with high efficacy (recognition threshold of 0.95) for the quantification of functional DREs in the promoters of mammalian and viral genes was utilized. In particular, a reduced susceptibility to TCDD of the gene encoding HIV-1 P248 was justified by the only potential DRE being present in the gag gene encoding the HIV-1 P24 protein, whereas the regulatory region of the CMV gene encoding IE gp/UL37 has five potent DREs, 1.65 kb/UL36 has six DREs, pp65 and pp71 each have seven DREs, and pp150 has ten DREs.[7] Therefore, TCDD in the picomolar range may activate in human cells the AhR:Arnt transcription factor that triggers CMV reactivation by binding to numerous promoter DREs within the immediate-early (IE) genes UL37 and UL36 [5,7] thus committing viruses to the lytic cycle.
Recent epidemiological data on the SARS-CoV-2 pandemic add to the role of bodily TCDD in the transactivation of CMV (measured by a very high rate of CMV seropositivity), as highly increased mortality rates were revealed in northern Italy, where 40+ years after the “Seveso industrial accident”, the TCDD plasma level in pre-exposed subjects was 15 times the level found in the general population, and the cumulative mortality rate during the first wave of COVID-19 was 4.5 times the rate of the rest of Italy (doi.org/10.3389/fpubh.2020.620416). Additionally, Arctic Native (AN) people have a TCDD body burden seven to 25 times that in the general population, as they consume dioxin-contaminated fat in seafood,5 and their COVID-19 mortality in persons aged 40-49 years is 2.2 times that among non-AN Alaskans (doi: 10.15585/mmwr.mm6949a3). In fact, in COVID-19, ARDS worsens clinical outcomes caused by secondary Herpesviridae infection, and CMV reactivation may be triggered by higher concentrations of TCCD in the respiratory system because lipid storm within the lungs of severe COVID-19 patients has been recently reported.(9) Due to its hydrophobic character (Ko/w: 7.05), TCDD partitions into inflammatory lipids in lung tissue, thus augmenting its local concentration. Additionally, if the recently shown AhR activation in macrophages infected with murine coronavirus(10) is applicable to human epithelial cells infected with SARS-CoV-2, this would further strengthen the assertion made in this Comment. Last, since the effect of the local dose of TCDD in extrahepatic tissues is determined by its binding to AhR, COVID-19 personal risk severity assessment of patients with ARDS might be contingent on the AhR affinity to TCDD, which varies nearly 15-fold among people in the general population.(5)
REFERENCES
1 Moss P. “The ancient and the new”: is there an interaction between cytomegalovirus and SARS-CoV-2 infection? Immunity & Aging 2020; 17:14. Published online May 27. doi: 10.1186/s12979-020-00185-x PMCID: PMC7251217; PMID: 32501397
2 Le Balc’h P and Pinceaux K Pronier C Seguin P et al. Herpes simplex virus and cytomegalovirus reactivations among severe COVID-19 patients. Critical Care 2020; 24:530. https://doi.org/10.1186/s13054-020-03252-3
3 Kadambari S, Klenerman P, Pollard AJ. Why the elderly appear to be more severely affected by COVID‐19: The potential role of immunosenescence and CMV. Rev Med Virol 2020; 30:ee2144. https://doi.org/10.1002/rmv.2144
4 Murayama T, Inoue M, Nomura T, Mori S, Eizuru Y. 2,3,7,8-Tetrachlorodibenzo-p-dioxin is a possible activator of human cytomegalovirus replication in a human fibroblast cell line. Biochem. Biophys. Res. Commun 2002; 296:651-6. doi: 10.1016/s0006-291x(02)00921-x
5 Wu JS, Shur IN, Tsyrlov IB. Current human body burden of dioxins might up-regulate DRE-containing cytomegalovirus linked to inflammation and malignancy pathways. Organohalogen Comp 2008; 70:1471-1474. ISSN: 1026-4892
6 Tsyrlov IB and Shur IN. Mechanistic insight into activation by dioxin-type xenobiotics of cancer-linked common human viruses and HIV-1 virus. Lancet Infect Dis 2008; 8(8):498. doi: 10.1016/70172-5
7 Tsyrlov IB, Shur IN, Oschepkov DY, Pokrovsky AG. Xenobiotical virology: new mechanistic concept of human viruses upregulation by body burden level of dioxins via AhR-mediated transcriptional pathway. Int J Infect Dis 2012; 16S:e115. http:dx.doi.org/10.1016/j.ijid2012.05.265
8 Tsyrlov, IB Pokrovsky AG. Stimulatory effect of the CYP1A1 inducer 2,3,7,8-tetrachlorodibenzo-p-dioxin on the reproduction of HIV-1 in human lymphoid cell culture. Xenobiotica 1993; 23:457-467. doi: 10.3109/00498259309057034
9 Archambault AS, Zaid Y, Rakotoarivelo V, Doré É, et al. Lipid storm within the lungs of severe COVID-19 patients: Extensive levels of cyclooxygenase and lipoxygenase-derived inflammatory metabolites. MedRxiv 2020; doi.org/10.1101/2020.12.04.20242115
10 Grunewald ME, Shaban MG, Mackin SR, Fehr AR, Perlman S. Murine coronavirus infection activates the aryl hydrocarbon receptor in an indoleamine 2,3-dioxygenase-independent manner, contributing to cytokine modulation and proviral TCDD-inducible PARP expression. J Virol 2020; 94:eo1743-19. https://doi.org/10.1128/JVI.01743-19Wikipedia article about Ilya B. Tsyrlov, the President of Xenotox, Inc.

Dear colleague, thank you for visiting the Xenotox, Inc. website - http://www.xenobiovir.com/ Hopefully, you found something that sparked interest. Please check back for future updates.
You can reach us either by e-mail: [email protected] or by phone: 1-914-439-1856, or by fax: 1-914-722-4247
We at Xenotox, Inc. express our appreciation to Mark Iandovka, M.S. for development and design of this website.